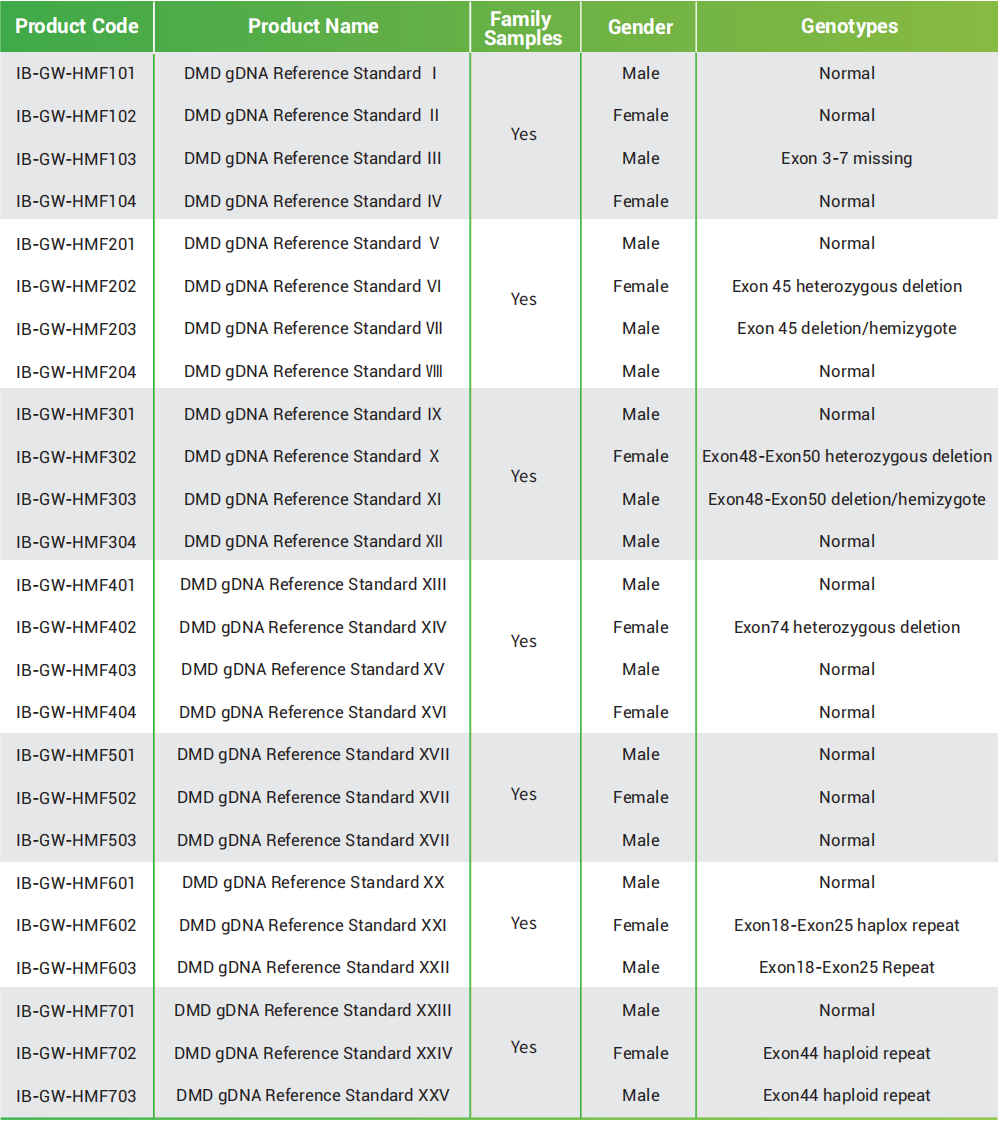
Duchenne muscular dystrophy (DMD) gDNA reference standard contains common DMD variants in family samples, including deletion and repeats among exons. These reference standards are derived from human cell lines and are ideal for performance validation, DMD assay kit verification and registration, and pedigree sample examinations on multiple nucleic acid detection and NGS platforms.
● Derived from human cell line
Mimic clinical samples
● Family sample
7 familie samples, including common DMD gene deletions and repeated mutations
● Wide range of applications
PCR-capillary electrophoresis, fluorescent PCR, multiple ligation-dependent probe amplification (MLPA), sequencing
● Product development and registration certificate
Third party reference standards for enterprises
● Routine quality control
Internal quality and external quality control for clinical testing
● Methodological comparison
Comparing for performance differences across platforms
● Performance evaluation of assays
Kit performance evaluation